Lantern Pharma (NASDAQ: LTRN), a biotech underdog, has announced promising preliminary results from its Phase 2 HARMONIC™ clinical trial, evaluating LP-300 in combination with standard chemotherapy for never-smokers with advanced non-small cell lung cancer (NSCLC). As reported earlier today, August 5, the study has shown an impressive 86% clinical benefit rate in the initial patient group, offering hope for a patient population with limited treatment options. The proportion of never-smoking patients with non-small cell lung cancer (NSCLC) has been significantly increasing globally over the past 30 years, from 15% in the 1970s to 33% in the 2000s. The high proportion of never smokers with NSCLC in East Asian countries is of particular note with Japan estimated to be 33 to 40% of new cases and Taiwan at over 50% of new cases. Lantern has received regulatory approval to initiate the LP-300 clinical trial in multiple Asian countries, and has started activation of sites in Japan and Taiwan, including the National Cancer Center in Tokyo, a globally recognized center of cancer research excellence.
AI in Drug Development
Artificial intelligence is revolutionizing the pharmaceutical industry, with Lantern Pharma’s RADR® platform leading the charge in oncology drug discovery and development. This AI-driven approach has significantly accelerated the process of identifying promising drug candidates and predicting their efficacy in specific patient populations. By analyzing over 100 billion data points, RADR® aids in validating mechanisms and uncovering insights that traditional methods might overlook. The platform’s success with LP-300 demonstrates the potential of AI to transform the cost, pace, and timeline of cancer therapy development, potentially bringing life-saving treatments to patients faster and more efficiently than ever before.
HARMONIC™ Study Results
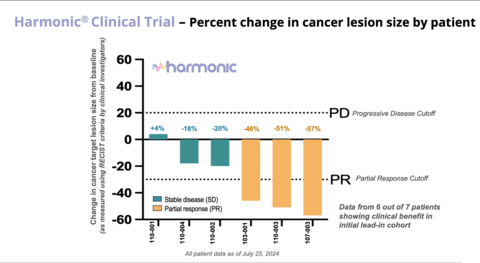
The HARMONIC™ Phase 2 study has yielded promising preliminary results for Lantern Pharma’s LP-300 drug candidate. In the initial safety lead-in cohort of 7 patients, 6 experienced clinical benefit, with 3 showing partial responses (average tumor size reduction of 51%) and 3 achieving stable disease (average tumor size reduction of 13%). The clinical benefit rate and disease control rate reached an impressive 86%, with an objective response rate of 43%. Notably, the study demonstrated no additional safety concerns, with no dose-limiting toxicities or treatment-related serious adverse events reported.
These encouraging results were observed across various patient profiles, including those with prior tyrosine kinase inhibitor treatments and low to intermediate tumor mutation burden. The study’s success is particularly significant given the lack of approved therapies specifically for never-smokers with NSCLC, a growing global patient population. As the trial progresses to its randomization and expansion phase, it aims to further assess the efficacy of LP-300 in combination with standard chemotherapy in this underserved patient group.
Future Implications and Milestones
Building on the promising preliminary results, Lantern Pharma is advancing the triplet combination regimen (LP-300 + pemetrexed + carboplatin) with the goal of improving outcomes for never-smokers with advanced NSCLC adenocarcinoma. The company is considering applying for Breakthrough Therapy designation if additional data confirms the initial findings. With no therapies specifically approved for never-smokers with NSCLC, this represents a significant clinical need with an estimated annual market potential exceeding $2 billion USD.
Key upcoming milestones include:
* Expansion of the Harmonic™ study to enroll up to 80 additional patients
* Review of interim data for Progression Free and Overall Survival after 30 clinical events
* Potential exploration of LP-300 in combination with other approved agents for cancer progression control
* Further utilization of the RADR® AI platform to uncover additional insights and targeted patient populations