
The MODD1 pump platform, developed by Modular Medical (NASDAQ: MODD), may play a significant role in controlling obesity and weight loss by offering a novel approach to delivering short-acting peptides as an alternative to long-acting GLP-1 therapies.
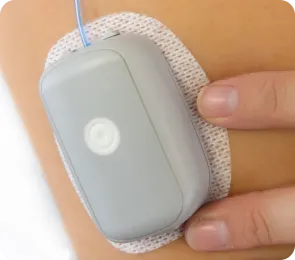
MODD1, Your Discreet Companion
Here’s how the MODD1 pump could potentially contribute to weight management:
1. Personalized delivery of short-acting peptides:
The MODD1 pump platform is being explored as a delivery system for FDA-approved, short-acting peptides[2][3]. This approach could provide a more tailored and tolerable solution for patients who struggle with long-acting GLP-1 therapies, particularly those with type 2 diabetes and obesity[3].
2. Addressing challenges with long-acting GLP-1 therapies:
Many patients prescribed GLP-1 drugs for weight loss discontinue usage within the initial weeks due to issues such as tolerability, cost, and inconsistent efficacy[3]. The MODD1 pump system aims to mitigate these problems by offering better dosage control and potentially enhancing the effectiveness of short-acting peptides.
3. Simplified dosing regimens:
Short-acting peptides have generally been abandoned as “too difficult to use” because they required multiple doses per day to be effective. The MODD1 pump technology could greatly simplify these dosing regimens by providing both a basal dose and boluses to control hunger[4].
4. Potential for improved glycemic control and weight loss:
The proof-of-concept study with Gubra A/S aims to determine whether an existing FDA-approved, short-acting peptide delivered from the MODD1 pump platform can provide comparable glycemic control and weight loss to long-acting GLP-1 drugs, while being more tolerable for patients[4].
5. User-friendly design:
The MODD1 pump is designed to be simple to learn and use, with features such as basal and bolus insulin delivery and a large prefill-ready reservoir[4]. This user-friendly approach could make it easier for patients to adhere to their treatment regimens, potentially leading to better outcomes in weight management.
6. Continuous therapy:
Unlike traditional injections, the pump system could provide continuous delivery of the short-acting peptides, potentially offering more consistent therapeutic effects throughout the day.
7. Data tracking and monitoring:
The MODD1 platform includes features for sharing insulin management data with healthcare providers[6], which could be extended to tracking weight loss progress and adjusting treatment plans accordingly.
Research Continues
While the use of the MODD1 pump for obesity control is still in the research phase, this innovative approach could potentially offer a new tool in the fight against obesity, especially for those who have found long-acting GLP-1 therapies challenging to use or ineffective. The ongoing proof-of-concept study will provide more insights into the feasibility and effectiveness of this approach in controlling obesity and facilitating weight loss.
On July 15, Modular Medical, Inc. (NASDAQ:MODD), an insulin delivery technology company seeking to launch the next generation of user-friendly and affordable insulin pump technology, today announced a proof-of-concept study with Gubra A/S (“Gubra”) in a high-fat, diet-induced obese (“DIO”) mouse model to explore the potential future use of the MODD1 pump platform to assist patients who struggle with tolerability, inconsistent efficacy, and cost of long acting GLP-1 therapies.

James (Jeb) Besser, CEO of Modular Medical (NASDAQ: MODD)
“A recent study published by Blue Health Intelligence using data from a national dataset of private insurers found that about half of all patients prescribed a GLP-1 drug for weight loss discontinued after the first 12 weeks, with approximately 30% discontinuing in the first four weeks,” commented Jeb Besser, Chief Executive Officer of Modular Medical. “These discontinuations appear to be due to a combination of tolerability, cost, and inconsistent efficacy. We suspect that short-acting peptides may mitigate many of these side effects and dosage swings by better modulating dosage, but these therapies have generally been abandoned as “too difficult to use” because they required multiple doses per day to be effective. We see the potential for such dosing regimes to be greatly simplified and even improved by the use of pump technology to provide both a basal dose and boluses to control hunger. Using Gubra’s gold-standard DIO mouse model, MODD will seek to determine whether an existing FDA approved, short-acting peptide delivered from a pump platform can provide a more personalized and more tolerable solution for patients who found long acting GLP-1 drugs too difficult to tolerate, while delivering comparable glycemic control and weight loss, specifically for people with type 2 diabetes and obesity.”

Michael Feigh, PhD, Vice President, Scientific Research & Sales of Gubra, commented, “We’re happy that our expertise and disease specific models are used in the assessment of Modular Medical’s novel approach to peptide therapy in diabetes and obesity.”
Modular Medical is focused on the delivery of therapeutics using patented technology with greater simplicity, lower cost and a differentiated form factor.

Paul DiPerna, Chairman and President of Modular Medical
“While long acting GLP-1 injectables have shown great results in the management of metabolic disease, we believe this research has the potential to help patients who would otherwise lose out on realizing those important clinical benefits,” commented Paul DiPerna, Chairman and President of Modular Medical. “Our simple to learn platform, basal and bolus features, and large prefill-ready reservoir make the MODD1 an ideal candidate for this potential application, once again furthering our mission of diabetes care for the rest of us.”
Citations:
[1] https://www.diabettech.com/insulin-pump/the-modular-medical-modd1-insulin-pump-what-do-we-know/
[2] https://www.accesswire.com/888097/modular-medical-announces-proof-of-concept-study-for-personalized-metabolic-therapy-utilizing-the-modd1-platform
[3] https://finance.yahoo.com/news/modular-medical-announces-proof-concept-120000997.html
[4] https://www.biospace.com/modular-medical-announces-proof-of-concept-study-for-personalized-metabolic-therapy-utilizing-the-modd1-platform
[5] https://www.beckershospitalreview.com/pharmacy/eli-lilly-weight-loss-drug-platform-draws-experts-concern.html
[6] https://www.modular-medical.com/modd1
[7] https://www.modular-medical.com/providers
[8] https://www.mdpi.com/1999-4923/16/7/944
[9] https://www.wallstreet-online.de/nachricht/18270618-modular-medical-announces-proof-of-concept-study-for-personalized-metabolic-therapy-utilizing-the-modd1-platform
[10] https://www.nbcnews.com/health/health-news/fda-approves-weight-loss-stomach-pump-aspireassist-combat-obesity-n592141




























































































































































































































