Indaptus Therapeutics (NASDAQ: INDP) has evolved from more than a century of immunotherapy advances. The Company’s novel approach is based on the hypothesis that efficient activation of both innate and adaptive immune cells and pathways and associated anti-tumor and anti-viral immune responses will require a multi-targeted package of immune system-activating signals that can be administered safely intravenously (i.v.). Indaptus’ patented technology is composed of single strains of attenuated and killed, non-pathogenic, Gram-negative bacteria producing a multiple Toll-like receptor (TLR), Nucleotide oligomerization domain (NOD)-like receptor (NLR) and Stimulator of interferon genes (STING) agonist Decoy platform. The product candidates are designed to have reduced i.v. toxicity, but largely uncompromised ability to prime or activate many of the cells and pathways of innate and adaptive immunity. Decoy product candidates represent an antigen-agnostic technology that have produced single-agent activity against metastatic pancreatic and orthotopic colorectal carcinomas, single agent eradication of established antigen-expressing breast carcinoma, as well as combination-mediated eradication of established hepatocellular carcinomas and non-Hodgkin’s lymphomas in standard pre-clinical models, including syngeneic mouse tumors and human tumor xenografts. In pre-clinical studies tumor eradication was observed with Decoy product candidates in combination with anti-PD-1 checkpoint therapy, low-dose chemotherapy, a non-steroidal anti-inflammatory drug, or an approved, targeted antibody. Combination-based tumor eradication in pre-clinical models produced innate and adaptive immunological memory, involved activation of both innate and adaptive immune cells, and was associated with induction of innate and adaptive immune pathways in tumors after only one i.v. dose of Decoy product, with associated “cold” to “hot” tumor inflammation signature transition. IND-enabling, nonclinical toxicology studies demonstrated i.v. administration without sustained induction of hallmark biomarkers of cytokine release syndromes, possibly due to passive targeting to liver, spleen, and tumor, followed by rapid elimination of the product. Indaptus’ Decoy product candidates have also produced significant single agent activity against chronic hepatitis B virus (HBV) and chronic human immunodeficiency virus (HIV) infections in pre-clinical models.
![]()
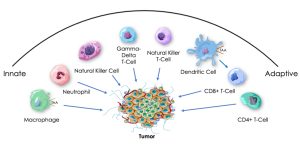
On April 11, Indaptus announced it was proud to unveil its poster at the 2024 Annual Meeting of the American Association for Cancer Research (AACR) in San Diego on Wednesday, April 10th. The American Association for Cancer Research (AACR) is the first and largest cancer research organization dedicated to accelerating the conquest of cancer. The Indpatus poster details mechanism of action data that demonstrates the Company’s Decoy platform successfully induces, matures or activates multiple immune cell types involved in anti-tumor responses. The latest findings significantly enhance the Company’s understanding of its “Decoy” platform technology, which uses killed, non-pathogenic bacteria engineered to activate the immune system to attack tumors. The study highlights the platform’s effectiveness in engaging key innate and adaptive immune cells, including, natural killer cells, natural killer T cells, dendritic cells, CD4+, and CD8+ T cells. In some settings, the platform also produced additive or synergistic activity in combination with IL-2, an approved cancer drug. Additionally, the data reveal that the Decoy platform may not only boost the immune system’s ability to recognize and kill tumor cells, but potentially also overcome a mechanism that suppresses the immune response. The results suggest that the Company’s Decoy bacteria can both directly and indirectly prime the immune system to more effectively fight cancer. Jeffrey Meckler, Indaptus’ Chief Executive Officer, added, “We are encouraged by the promising results observed in our preclinical studies and our ongoing Phase 1 clinical trial. The recognition and validation from prestigious organizations such as the AACR, coupled with the support and insights we are receiving from medical experts, partners and investors at the conference, inspire us to continue advancing our technology and demonstrating its significant therapeutic potential for the treatment of solid tumors.” The full poster can be accessed on the Indaptus Therapeutics website by clicking here.

Dr. Michael Newman, Indaptus’ Founder, Chief Scientific Officer
Dr. Michael Newman, Indaptus’ Founder, Chief Scientific Officer, and lead author, commented, “The new data are consistent with our preclinical animal tumor model studies and provide evidence for our hypothesis that patented Decoy bacteria can activate a wide range of innate and adaptive human immune cells involved in fighting tumors. This aligns with what we’ve observed in our ongoing Phase 1 clinical trial of Decoy20 – broad immune activation, as evidenced by transiently increased levels of many key cytokines and chemokines following single dose administration. These findings bolster our confidence in Decoy20’s potential as a multifaceted immunotherapy.”
On March 4, 2024, Indaptus Therapeutics announced positive results from the second cohort of its Phase 1 INDP-D101 trial. Patients continue to exhibit a broad immune response similar to the first cohort. The preliminary results of this study were reviewed by the Company and an independent Safety Review Committee. Based on this review, it was recommended that the Company continue the trial and enroll patients for multiple doses of its lead therapeutic candidate, Decoy20. The company has immediately started screening potential patients. The primary goal of the next stage of the trial is to determine the safety of Decoy20 when administered multiple times to the same patient, and to begin to examine the efficacy across multiple types of cancer. In animal models, Decoy20 was shown to be safe in several multiple dosing schedules.

Jeffrey Meckler, CEO, Indaptus Therapuetics, Inc. (NASDAQ: INDP)
Jeffrey Meckler, Indaptus’ Chief Executive Officer, commented, “We have now confirmed the safety requirements necessary to advance our Phase 1 trial of Decoy20 to multi-dosing. The ability to do so is based on positive safety outcomes in the single dose regimen as well as the anti-cancer activity we observed from multi-dosing in our pre-clinical models.”

Shares of Indaptus Therapeutics (NASDAQ: INDP) closed at $2.75 on Thursday and up a whopping +49.86% year-to-date. Check out INDP’s stock quote today.
The AACR: Driving Progress Against Cancer
What’s the Indaptus Timeline?
Dr. Roger Waltzman, Chief Medical Officer, shares the Indaptus journey with #Decoy20.
Indaptus Science & Pipeline
Novel Insights. Novel Therapies.
Historically, we know that tumor regression has been observed in the presence of bacterial infection. Indaptus knows that bacteria contain immune system danger signals, called pathogen-associated molecular patterns (PAMPs), that collectively can activate all of the cellular components of our innate and adaptive immune pathways. PAMPs are recognized by receptors, such as Toll-like (TLR), NOD, STING and RIG-I, that are found on and involved in activation of many different innate and adaptive immune cells.
The Indaptus platform is based on the hypothesis that highly efficient anti-tumor immunotherapy will require safe activation of both innate and adaptive cellular immunity in both tumors and immune organs, and that this might be achieved with a multi-targeted package of bacterial PAMPs, in the form of attenuated and killed, intact but non-pathogenic bacteria delivered intravenously. While current therapies are increasingly becoming more and more personalized and costly, we are advancing an approach designed to be widely accessible, with broad anti-tumor and anti-viral activity not dependent on the targeting of specific tumor or viral antigens.
Learn more about Indaptus’ science and pipeline by clicking here.
Indaptus Publications and Presentations
April 5-10, 2024 – AACR Poster Presentation
November 1-5, 2023 – Society for Immunotherapy of Cancer (SITC)
May 9-11, 2023 – 4th STING & TLR Targeting Therapies Summit
- Pre-Clinical Anti-HBV Activity with a Passively Targeted, Multi-TLR, NOD and STING Agonist
- Breaking the Toxicity Barrier: Tumor Eradication in Pre-Clinical Models by Systemic Administration of a Multi-TLR, NOD and STING Agonist
April 2023 – AACR Poster Presentation
- A systemically administered killed bacteria-based immune receptor agonist for pulsed anti-tumor immunotherapy
April 25-27, 2022 – Chronic HBV Drug Development
- Driving Pre-Clinical Anti-HBV Activity With a Novel Multi-TLR Agonist Therapeutic Vaccine
STING & TLR-Targeted Therapies Summit 2022
- Eradication of Established Tumors with Induction of Innate & Adaptive Immunological Memory in Multiple Preclinical Models with Systemically Administered Decoy Bacteria, a Multi-TLR Agonist Therapeutic Vaccine
May 25-27, 2021 – STING & TLR-Targeting Therapies Summit – Virtual Presentation
- Combination Systemic Therapy with a Multiple TLR Agonist Safely Eradicates Established Tumors with Induction of Innate and Adaptive Immunological Memory
2018 Meeting of CRI-CIMT-EATI-AACR – Poster Presentation
- Development and pre-clinical efficacy characterization of a systemically administered multiple Toll-like receptor (TLR) agonist for anti-tumor immunotherapy
LEARN MORE ABOUT INDAPTUS NOW….

Indaptus CEO presented in an open Q&A on Tribe Public’s Webinar titled, “Fully Engaging The Human Immune System To Cure Disease” on Wednesday, March 20, 2024. You can now watch the event video at the Tribe Public YouTube Channel at this link.