company profile
Indaptus Therapeutics has evolved from more than a century of immunotherapy advances. The Company’s novel approach is based on the hypothesis that efficient activation of both innate and adaptive immune cells and pathways and associated anti-tumor and anti-viral immune responses will require a multi-targeted package of immune system-activating signals that can be administered safely intravenously (i.v.). Indaptus’ patented technology is composed of single strains of attenuated and killed, non-pathogenic, Gram-negative bacteria producing a multiple Toll-like receptor (TLR), Nucleotide oligomerization domain (Nod)-like receptor (NLR) and Stimulator of interferon genes (STING) agonist Decoy platform. The products are designed to have reduced i.v. toxicity, but largely uncompromised ability to prime or activate many of the cells and pathways of innate and adaptive immunity. Decoy products represent an antigen-agnostic technology that have produced single-agent activity against metastatic pancreatic and orthotopic colorectal carcinomas, single agent eradication of established antigen-expressing breast carcinoma, as well as combination-mediated eradication of established hepatocellular carcinomas and non-Hodgkin’s lymphomas in standard pre-clinical models, including syngeneic mouse tumors and human tumor xenografts. In pre-clinical studies tumor eradication was observed with Decoy products in combination with anti-PD-1 checkpoint therapy, low-dose chemotherapy, a non-steroidal anti-inflammatory drug, or an approved, targeted antibody. Combination-based tumor eradication in pre-clinical models produced innate and adaptive immunological memory, involved activation of both innate and adaptive immune cells, and was associated with induction of innate and adaptive immune pathways in tumors after only one i.v. dose of Decoy product, with associated “cold” to “hot” tumor inflammation signature transition. IND-enabling, nonclinical toxicology studies demonstrated safe i.v. administration without sustained induction of hallmark biomarkers of cytokine release syndromes, possibly due to passive targeting to liver, spleen, and tumor, followed by rapid elimination of the product. Indaptus’ Decoy products have also produced significant single agent activity against chronic hepatitis B virus (HBV) and chronic human immunodeficiency virus (HIV) infections in pre-clinical models.
The Indaptus Goal is Simple: to Cure Disease
With the ability to harness both the body’s innate and adaptive immune responses, we believe we are uniquely positioned to revolutionize the treatment of cancer and certain infectious diseases.
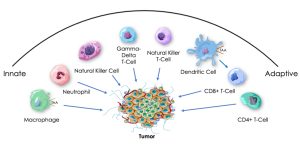
Building on the observation that tumor regression can occur in the setting of bacterial infection, we have developed a proprietary platform that exploits bacteria’s natural ability to activate both innate and adaptive cellular immune pathways. Leveraging our novel insights into the levels and ratios of activating signals needed to safely elicit a broad immune response, we are creating and advancing a pipeline of proprietary, attenuated and killed non-pathogenic gram-negative bacterial candidates designed to be widely accessible, with broad anti-tumor and anti-viral activity.
Their candidates, including their lead candidate Decoy20, have demonstrated, in pre-clinical studies, the ability to elicit single-agent activity and durable anti-tumor responses in the combination setting against colorectal, hepatocellular, pancreatic carcinoma, and non-Hodgkin’s lymphoma in standard pre-clinical models. We believe that our Decoy candidates are unique in their ability to synergize with each of a wide variety of existing therapies, including checkpoint therapy and targeted antibodies, to produce durable anti-tumor responses against established tumors, with induction of both innate and adaptive immunological memory.
Based on these positive pre-clinical results to date, they have initiated our first Phase 1 clinical trial in December 2022 and dosed our first patient in March 2023.
Management

Jeffrey Meckler, CEO, Indaptus Therapuetics, Inc. (NASDAQ: INDP)
Jeffrey Meckler currently serves as Chief Executive Officer, bringing more than 30 years of financial and healthcare leadership experience to the company. Most recently, Jeff was the CEO of Intec Pharma, and prior to that, CEO of Cocrystal Pharma, transforming it from a research company into a clinical and development company. Earlier in his career, Jeff was managing director of the Andra Group, a life sciences consulting firm, and acted as a director and interim CEO of Cypress Bioscience after its acquisition by Royalty Pharma. Jeff started his career at Pfizer, where he held a series of positions in manufacturing systems, market research, business development, strategic planning and corporate finance, which included playing a significant role in acquisitions and divestitures. He has also served as a director of QLT, Inc., Cocrystal Pharma, ClearFarma USA, Kyalin Bioscience, and Alveolus, and currently serves as director of Travere Therapeutics, where he also previously served as Chairman. Jeff is the past President and continues to serve on the Board of Children of Bellevue, a non-profit organization focused on advocating and developing pediatric programs at Bellevue Hospital Center. He holds a B.S. in industrial management, an M.S. in industrial administration from the Tepper School of Business at Carnegie Mellon University, and a J.D. from Fordham University’s School of Law.

A founder of the company, Dr. Michael Newman currently serves as our Chief Scientific Officer. Most recently, he was Founder and CEO of Decoy Biosystems, where he developed the technology that serves as the foundation of Indaptus. With more than 35 years of experience carrying out and managing oncology drug discovery through early development in academia and at pharmaceutical and biotechnology companies, Michael has also served as a consultant to ~35 companies, assisting with target identification and prioritization, management of R&D, fundraising, and in/out-licensing. His previous positions include faculty appointments in biochemistry at Brandeis University and the Roche Institute of Molecular Biology, Senior Associate Director of Oncology at Sandoz Pharmaceuticals (world-wide head of Cancer Biology), Executive Director of Oncology at Novartis Pharmaceuticals (Head of Cancer Biology in the U.S.), and senior management positions at several Biotechnology companies, where he also managed drug discovery programs in inflammation, diabetes, and infectious disease. Michael received a Bachelor’s degree in biology from the University of California at San Diego, a Ph.D. in cell and developmental biology from Harvard Medical School (National Science Foundation Pre-doctoral Fellow) and carried out post-doctoral research at Cornell University.
vista's key points
- Indaptus in headquartered in New York City & their stock trades on the Nasdaq under the symbol “INDP.”
- Building upon the long-standing observation of tumor regression in the setting of bacterial infection, Indaptus has developed a unique and patented approach designed to exploit bacteria’s natural ability to fully engage the human immune system and cure disease.
- Indaptus' novel insights have enabled the Company to create proprietary, attenuated and killed non-pathogenic gram-negative bacteria that have demonstrated broad anti-tumor and anti-viral activity in pre-clinical models to date.
- With the ability to harness both the body’s innate and adaptive immune responses, Indaptus believes they are uniquely positioned to revolutionize the treatment of cancer and certain infectious diseases.
- Indaptus is leveraging their novel insights into the levels and ratios of activating signals needed to safely elicit a broad immune response, they are creating and advancing a pipeline of proprietary, attenuated and killed non-pathogenic gram-negative bacterial candidates designed to be widely accessible, with broad anti-tumor and anti-viral activity.
- Indaptus therapeutic candidates, include their lead candidate Decoy20 that has have demonstrated, in pre-clinical studies, the ability to elicit single-agent activity and durable anti-tumor responses in the combination setting against colorectal, hepatocellular, pancreatic carcinoma, and non-Hodgkin’s lymphoma in standard pre-clinical models.
- Indaptus believes that Decoy candidates are unique in their ability to synergize with each of a wide variety of existing therapies, including checkpoint therapy and targeted antibodies, to produce durable anti-tumor responses against established tumors, with induction of both innate and adaptive immunological memory.
- Based on Indaptus' positive pre-clinical results to date, they have initiated their first Phase 1 clinical trial in December 2022 and dosed their first patient in March 2023.
- The Company announced the completion of the first cohort of its INDP-D101 trial and receipt of authorization from its Safety Review Committee to proceed into the second cohort of the Phase 1 trial.
- A compound from the Company’s Decoy platform was presented in a poster titled, “A systemically administered killed bacteria-based multiple immune receptor agonist for pulsed anti-tumor immunotherapy ,” at the American Association for Cancer Research Conference 2023. The poster highlighted that Decoy10 demonstrated 90% reduction of LPS-endotoxin activity and use of 100% killed, non-pathogenic bacteria.
- The Company received patent allowances for its Decoy immunotherapy platform in Brazil and India. The Indian patent allowance brought the number of countries in which the Company holds patent protection to 32.
- On March 4, Indaptus announced positive results from the second cohort of its Phase 1 INDP-D101 trial. Patients continue to exhibit a broad immune response similar to the first cohort. The preliminary results of this study were reviewed by the Company and an independent Safety Review Committee. Based on this review, it was recommended that the Company continue the trial and enroll patients for multiple doses of its lead therapeutic candidate, Decoy20. The company has immediately started screening potential patients.
- On March 13, Indaptus reported that as of December 31, 2023, the Company had cash and cash equivalents of $13.4 million. The Company expects that its current cash and cash equivalents will support its ongoing operating activities through the third quarter of 2024. This cash runway guidance is based on the Company’s current operational plans and excludes any additional funding (including aggregate gross proceeds of $0.3 million from sales of shares of the Company’s common stock under its ATM program in March 2024) and any business development activities that may be undertaken. Indaptus continues to assess all financing options that would support its corporate strategy.
- On April 11 Indaptus announced that it unveiled its poster at the 2024 Annual Meeting of the American Association for Cancer Research (AACR) in San Diego on Wednesday, April 10th. The poster details mechanism of action data that demonstrates the Company’s Decoy platform successfully induces, matures or activates multiple immune cell types involved in anti-tumor responses.
VISTA'S PROGRESS REPORT
Coming Soon
vista's key points
- Indaptus in headquartered in New York City & their stock trades on the Nasdaq under the symbol “INDP.”
- Building upon the long-standing observation of tumor regression in the setting of bacterial infection, Indaptus has developed a unique and patented approach designed to exploit bacteria’s natural ability to fully engage the human immune system and cure disease.
- Indaptus' novel insights have enabled the Company to create proprietary, attenuated and killed non-pathogenic gram-negative bacteria that have demonstrated broad anti-tumor and anti-viral activity in pre-clinical models to date.
- With the ability to harness both the body’s innate and adaptive immune responses, Indaptus believes they are uniquely positioned to revolutionize the treatment of cancer and certain infectious diseases.
- Indaptus is leveraging their novel insights into the levels and ratios of activating signals needed to safely elicit a broad immune response, they are creating and advancing a pipeline of proprietary, attenuated and killed non-pathogenic gram-negative bacterial candidates designed to be widely accessible, with broad anti-tumor and anti-viral activity.
- Indaptus therapeutic candidates, include their lead candidate Decoy20 that has have demonstrated, in pre-clinical studies, the ability to elicit single-agent activity and durable anti-tumor responses in the combination setting against colorectal, hepatocellular, pancreatic carcinoma, and non-Hodgkin’s lymphoma in standard pre-clinical models.
- Indaptus believes that Decoy candidates are unique in their ability to synergize with each of a wide variety of existing therapies, including checkpoint therapy and targeted antibodies, to produce durable anti-tumor responses against established tumors, with induction of both innate and adaptive immunological memory.
- Based on Indaptus' positive pre-clinical results to date, they have initiated their first Phase 1 clinical trial in December 2022 and dosed their first patient in March 2023.
- The Company announced the completion of the first cohort of its INDP-D101 trial and receipt of authorization from its Safety Review Committee to proceed into the second cohort of the Phase 1 trial.
- A compound from the Company’s Decoy platform was presented in a poster titled, “A systemically administered killed bacteria-based multiple immune receptor agonist for pulsed anti-tumor immunotherapy ,” at the American Association for Cancer Research Conference 2023. The poster highlighted that Decoy10 demonstrated 90% reduction of LPS-endotoxin activity and use of 100% killed, non-pathogenic bacteria.
- The Company received patent allowances for its Decoy immunotherapy platform in Brazil and India. The Indian patent allowance brought the number of countries in which the Company holds patent protection to 32.
- On March 4, Indaptus announced positive results from the second cohort of its Phase 1 INDP-D101 trial. Patients continue to exhibit a broad immune response similar to the first cohort. The preliminary results of this study were reviewed by the Company and an independent Safety Review Committee. Based on this review, it was recommended that the Company continue the trial and enroll patients for multiple doses of its lead therapeutic candidate, Decoy20. The company has immediately started screening potential patients.
- On March 13, Indaptus reported that as of December 31, 2023, the Company had cash and cash equivalents of $13.4 million. The Company expects that its current cash and cash equivalents will support its ongoing operating activities through the third quarter of 2024. This cash runway guidance is based on the Company’s current operational plans and excludes any additional funding (including aggregate gross proceeds of $0.3 million from sales of shares of the Company’s common stock under its ATM program in March 2024) and any business development activities that may be undertaken. Indaptus continues to assess all financing options that would support its corporate strategy.
- On April 11 Indaptus announced that it unveiled its poster at the 2024 Annual Meeting of the American Association for Cancer Research (AACR) in San Diego on Wednesday, April 10th. The poster details mechanism of action data that demonstrates the Company’s Decoy platform successfully induces, matures or activates multiple immune cell types involved in anti-tumor responses.
exclusive content
Vista Partners creates exclusive content based on the ongoing research of companies included in the VP Watchlist.
vp watchlist
The VP Watchlist contains current coverage companies that deserve consideration for short term and long term portfolio additions.
recent news
-
Indaptus Therapeutics, Inc. Announces Additional Sale of...
1 July 2025 | 12:14 pm
-
Indaptus Therapeutics Announces Reverse Stock Split
25 June 2025 | 11:00 am
-
Indaptus Therapeutics, Inc. Announces Sale of $2.3...
13 June 2025 | 12:00 pm
Interviews
Stay Informed. Stay Competitive with FREE Insights on the Stock Market, Dow 30 & Emerging Opportunities.
Get Free Email Updatesvideos
- Indaptus Therapeutics’ CMO Roger Waltzman Explains How They Manage Side Effects of Decoy20 – $INDP
- “Diabetes, Obesity, GLP-1, & The MODD-1 Opportunity” – Modular Medical (Nasdaq: MODD) CEO Jeb Besser
- Starlight Therapeutics – Born from AI, Lighting the Way in CNS Cancer Treatment
- Lantern Pharma | Second Quarter 2024 Operating & Financial Results Conference Call
- “Exploring The Rapid Rise Of Osteoarthritis” With Eupraxia’s (EPRX) CEO Dr. James Helliwell
- “Diabetes Care For The Rest Of Us!” – Modular Medical (MODD)
- Lantern (LTRN) CEO Panna Sharma Presents “How Artificial Intelligence Is Crushing Drug Discovery Times & Costs In Cancer”










































