
Reportedly, Intel Corporation (INTC) is currently engaged in discussions with Arm, a subsidiary of SoftBank Group Corp, to become a significant investor in Arm’s upcoming initial public offering (IPO) of shares. According to a report, Arm intends to list its shares on Nasdaq later this year, with the aim of raising $8 to $10 billion in capital.
Arm’s chip designs are widely utilized by major semiconductor companies such as Intel, AMD, Nvidia, and Qualcomm. The potential effects of an IPO investment from these companies on Arm’s existing business partnerships remain uncertain at this time.
Intel Corporation (INTC) seeks to expand the boundaries of technology to provide the most amazing experience possible while designing, manufacturing, and selling integrated digital technology globally. To learn more about Intel Corporation (INTC) and to track its progress please visit the Vista Partners Intel Corporation Coverage Page.

If you liked this story please consider, visiting the Atossa Therapeutics (ATOS) dedicated page at Vista Partners to learn about the Seattle-based biotech firm that is seeking to develop innovative medicines in areas of significant unmet medical need in oncology with a current focus on breast cancer and lung injury caused by cancer treatments.
Vista Partners LLC (”Vista”) is a California Registered Investment Advisor based in San Francisco. Vista delivers timely and relevant insights via the website: www.vistapglobal.com with daily stories, weekly market updates, monthly macroeconomic newsletters, podcasts, & Vista’s proprietary equity and market research to help you stay informed and stay competitive. Vista’s mission is to invest partner capital while arming investors with a comprehensive global financial perspective across all market sectors. Vista also seeks to provide select issuers with actionable advice regarding fundamental development, corporate governance, and capital market directives.
Stay Informed! Stay Competitive! Please join us at Vista Partners, receive our FREE email updates throughout the week, and view our exclusive content and research.


Today, Lantern Pharma Inc. (NASDAQ: LTRN, $5.83, +7.96%), a clinical-stage biopharmaceutical company using its proprietary RADR® artificial intelligence (“AI”) and machine learning (“ML”) platform to transform the cost, pace, and timeline of oncology drug discovery and development, announced that the U.S. Food and Drug Administration (FDA) has cleared the investigational new drug (IND) application for LP-184, which is being developed for multiple advanced solid tumors and central nervous system (CNS) cancers. The first-in-human Phase 1A trial for LP-184 is anticipated to launch and dose its first patient during the current quarter. LP-184 is a novel, synthetically-lethal, small molecule that has been developed using insights from Lantern’s AI platform, RADR®. Lantern has been granted multiple Orphan Drug Designations by the FDA for LP-184 in pancreatic cancer, malignant gliomas, and atypical teratoid rhabdoid tumors (ATRT); in addition the FDA granted Rare Pediatric Disease Designation granted for LP-184 in ATRT. The cancer indications being pursued for LP-184 are estimated to have an annual market potential of $11-13 billion; $6-7 billion for solid tumors and $5-6 billion for brain and CNS cancers.
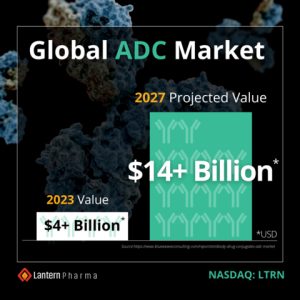
On June 8, Lantern announced it will be leveraging its AI platform, RADR®, in a research collaboration with Bielefeld University (Bielefeld, Germany) to develop antibody-drug conjugates (ADCs) with high therapeutic and antitumor potential. The collaboration will leverage insights from Lantern’s recently developed RADR® AI ADC module in combination with research from Professor Norbert Sewald, Ph.D., the principal investigator for Bielefeld and leader of Magicbullet::reloaded, a European consortium focused on developing novel drug delivery mechanisms, including ADCs. Outcomes from the collaboration are expected to pave the way for next-generation ADCs and other drug conjugates that are designed using AI and that can be developed with potentially higher efficacy, at a faster pace, and with significantly reduced costs. The collaboration with Bielefeld University (Germany) will be led by Professor Norbert Sewald, Ph.D., a leading expert in the synthesis of cryptophycins, development of ADCs, and the coordinator of the “Magicbullet::reloaded” consortium. Lantern is receiving an exclusive and worldwide option to license intellectual property from Bielefeld University related to the collaboration and IP generated from the collaboration. The global ADC market is currently over $4.0 billion and is projected to reach $14.0 billion by 2027.






















































































