The global insulin pump market is experiencing significant growth, driven by technological advancements and increasing diabetes prevalence. As reported by Grand View Research, the market was valued at USD 6.05 billion in 2023 and is projected to grow at a compound annual growth rate of 8.22% from 2024 to 2030, reflecting the rising adoption of innovative diabetes management solutions.
Modular Medical’s Manufacturing Move
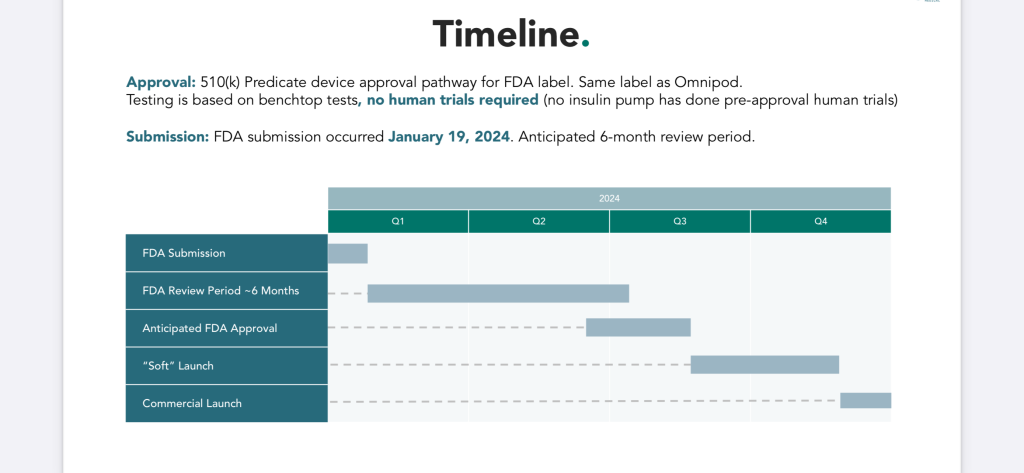
Modular Medical’s FDA Timeline Slide from their powerpoint that was updated Friday, August 2.¬†
 
 
Phillips Medisize Collaboration
Phillips Medisize, a Molex company, has been a crucial partner in developing Modular Medical’s platform product, supply chain, and manufacturing operations. Their collaboration extends beyond basic manufacturing, providing expertise in injection molding, packaging, electronics design, and assembly operations. This partnership has been instrumental as Modular Medical transitions from pre-commercial production to high-volume device manufacturing. The collaboration leverages Phillips Medisize’s global and diversified supplier base, enabling the design and development of manufacturing capabilities specifically tailored for the MODD1 Insulin Delivery System. Phillips Medicine was founded in 1964 in Phillips, WI, employs¬†6 thousand employees worldwide, has¬†24 manufacturing locations in 11 countries, including 5 R&D centers globally
2.5M+ square feet of manufacturing space, & maintains Class 7 & 8 cleanrooms and tool building sites.
Insulin Pump Market Growth
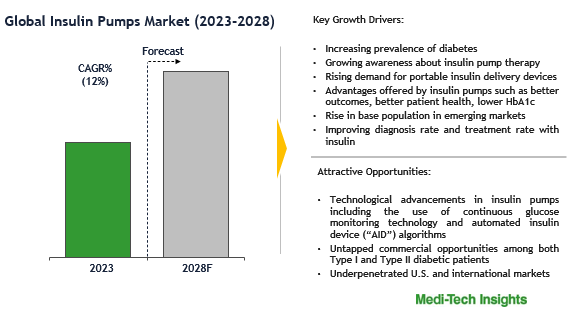
The insulin pump market is experiencing robust growth, with a valuation of USD 6.05 billion in 2023 and a projected compound annual growth rate (CAGR) of 8.22% from 2024 to 2030. This expansion is driven by technological advancements, increasing diabetes prevalence, and a growing elderly population. Key players in the market include Medtronic (MDT), Abbott Laboratories (ABT), Roche Diagnostics & Tandem Diabetes Care, Inc (TNDM). The global diabetes care devices market is expected to reach USD 52.34 billion by 2029, growing at a CAGR of 12.22% from 2024 to 2029. This growth is fueled by rising healthcare spending, increased awareness about diabetes management, and the development of innovative devices like continuous glucose monitors and automated insulin delivery systems.
Trends in Diabetes Care
Recent advancements in diabetes care technology have led to the development of innovative devices like the t:slim X2 Insulin Pump with Control-IQ Technology from Tandem Diabetes Care, which integrates with Dexcom (DXCM) G6 & G7 CGM to predict and prevent hypoglycemia without finger sticks. The FDA has also approved the world’s smallest durable automated insulin delivery system, the Tandem Mobi, for ages 6 and up, which is fully controllable from a mobile app. Other notable innovations include the FDA-cleared Beta Bionics iLet Bionic Pancreas, which uses an adaptive closed-loop algorithm initialized only with the user’s body weight, eliminating the need for manual adjustments of insulin pump settings.¬†Other cutting-edge developments include smart insulin pens with connectivity features, implantable devices for long-term medication delivery, and advancements in non-invasive insulin delivery methods such as oral and transdermal routes. As the global insulin pump market continues to grow, projected to reach $10.3 billion by 2032, these innovative technologies are poised to significantly enhance diabetes care and management.¬†These developments reflect a growing trend towards more user-friendly, efficient, and technologically advanced diabetes management solutions, addressing the needs of the estimated 29.7 million people in the U.S. diagnosed with diabetes as of 2021.
MODD1 Is Making FDA Progress
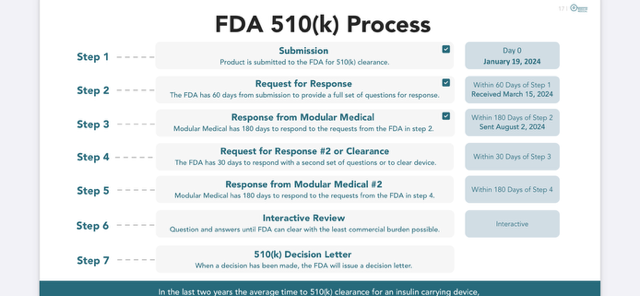
On Friday, August, 2, 2024, Modular Medical confirmed that they had taken the next step in their FDA process where they submitted their response to The FDA’s questions. The FDA has 30-days from their August 2nd submission to respond with a second set of questions or to clear the device.  See the detailed process outlined above from Modular Medical’s presentation that was updated this past Friday. Also see, the Modular Medical Timeline presentation slide that depicts a timeline for the FDA Clearance process for 2024.
Gain Further Insights From The CEO of Modular Medical…
On Friday, August 9, our sister organization, Tribe Public (www.TribePublic.com), will be host a brief CEO Presentation and Q&A Webinar-based event at 8:30am PT /11:30am ET with Jeb Besser, CEO of Modular Medical (NASDAQ: MODD). The event is titled¬†“Diabetes Care Innovator Modular Medical Takes Next Steps.”
You may register for this free event at MODDAugust92024.TribePublic.com and submit your questions ahead of the event at research@tribepublic.com or during the event via the zoom chat feature.