Q2 2025 Financial and Business Momentum
GeoVax Labs (NASDAQ: GOVX) is set to deliver its second quarter 2025 results on Monday, July 28, promising insights into a period of robust growth and scientific achievement.
Key EMA Regulatory Milestone
A breakthrough for GeoVax in 2025 is the favorable regulatory advice received from the European Medicines Agency (EMA). The EMA has indicated that a single, well-structured Phase 3 immuno-bridging trial might suffice to support a Marketing Authorization Application for GeoVax’s Mpox and smallpox vaccine, potentially bypassing the longer Phase 1 and 2 requirements. This development accelerates the pathway to approval, offering a substantial time advantage both for the company and for public health responses to emerging infectious disease threats.
Pipeline Strategy and Scientific Diversification
GeoVax continues building a diversified portfolio beyond Mpox, with clinical initiatives targeting COVID-19 and oncology. The GEO-CM04S1 vaccine is progressing in several Phase 2 studies, aiming to serve both healthy and immunocompromised individuals. In oncology, Gedeptin® is advancing as a treatment for head and neck cancers in combination with immunotherapy. Independent studies highlight the benefits of a multi-pronged vaccine approach, emphasizing the importance of non-mRNA platforms like GeoVax’s MVA-based technology.
Sell-Side Analyst Views and Price Targets
Analyst sentiment remains notably positive for GeoVax. Recent sell-side reports assign an average 12-month price target of $8.88 to $10.38 per share, implying over 1,000% upside from recent levels. All covering analysts rate GeoVax a “Buy” or better, with several designating it as a “Strong Buy.”
Key Takeaways
- Regulatory Advancement: EMA’s positive feedback could significantly shorten the vaccine approval timeline in Europe.
- Pipeline Strength: Progress continues across both infectious disease and oncology indications.
- Market Sentiment: Analysts are highly bullish, projecting major upside for GeoVax shares and reiterating strong buy recommendations.
GeoVax’s momentum in regulatory, scientific, and financial arenas makes it one of the more intriguing biotech stories for the remainder of 2025, with multiple catalysts in play for investors, clinicians, and the broader healthcare community.
Sources:
1. finance.yahoo
finance.yahoo.com/news/geovax-re…
GeoVax to Report Second Quarter 2025 Financial Results and …
2. marketbeat.com
marketbeat.com/earnings/repor…
GeoVax Labs Q2 2025 Earnings Report – MarketBeat
GeoVax Labs announced their Q2 2025 earnings on 8/5/2025. View GOVX’s earnings results at MarketBeat.

3. nasdaq
nasdaq.com/market-activit…
GeoVax Labs, Inc. Common Stock (GOVX) Earnings Report Date
4. ainvest
ainvest.com/stocks/NASDAQ-…
GOVX Earnings: GeoVax Labs Past Reports & EPS – AInvest
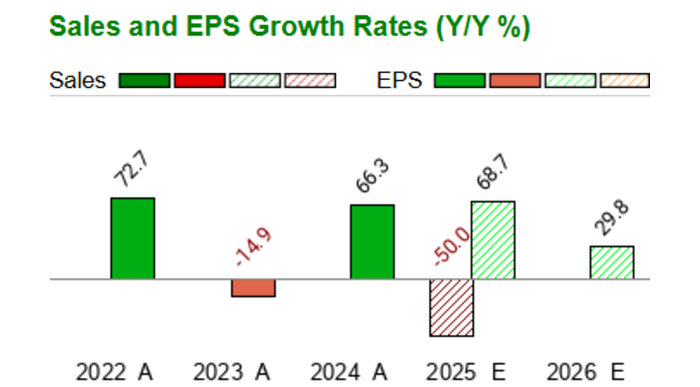
GOVX Q2 2025 Earnings Preview. GeoVax Labs (GOVX) expects to report earnings on Aug 5, 2025, with estimated revenue of 533.33K (YoY +77.38%), and EPS at …
5. Linkedin
linkedin.com/posts/newsramp…
GeoVax Labs, Inc. | NewsRamp™ – LinkedIn
GeoVax Labs, Inc. has secured a significant regulatory milestone with the European Medicines Agency (EMA), potentially accelerating its GEO-MVA vaccine development pathway for Mpox and smallpox prevention. The EMA’s Committee for Medicinal Products for Human Use (CHMP) provided groundbreaking guidance that could streamline the company’s regulatory approval process. Notably, the agency confirmed that a single, robust Phase 3 immuno-bridging trial against the approved MVA vaccine (Imvanex) would be sufficient to support a Marketing Authorization Application. This strategic breakthrough eliminates multiple traditional development steps, potentially expediting vaccine availability. David Dodd, GeoVax’s Chairman and CEO, emphasized the critical timing, highlighting the World Health Organization’s recent fourth declaration of Mpox as a Public Health Emergency of International Concern. The guidance addresses urgent global health needs, particularly with Mpox transmission escalating across 25 African countries and emerging in multiple continents. GeoVax’s approach offers a promising alternative to current vaccine solutions, with plans to transition to a scalable, cost-effective manufacturing platform. By potentially providing an additional vaccine source, GeoVax aims to strengthen global health resilience during a period of significant infectious disease challenges. The company remains committed to working with global regulators to ensure vaccine access and manufacturing transparency. This regulatory advancement represents a potential game-changer in infectious disease preparedness and vaccine development strategies.

6. GuruFocus
gurufocus.com/news/2929167/g…
GeoVax Labs (GOVX) Gains EMA Approval for Phase 3 Vaccine Trial
GeoVax Labs, trading under the ticker GOVX, has announced a significant milestone in its vaccine development program. The company has secured positive feedback
7. GeoVax, Inc.
geovax.com/investors/pres…
GeoVax Receives Favorable European Regulatory Guidance …
Confirms Single Phase 3 Immuno-Bridging Trial Sufficient to Evaluate Efficacy and to Support a Marketing Authorization Application (MAA) For Vaccination against Mpox and Smallpox ATLANTA, GA – June 16, 2025 – GeoVax Labs, Inc. (Nasdaq: GOVX), a
8. Newsworthy.ai
newsworthy.ai/curated/geovax…
GeoVax Advances Toward European Approval for Mpox Vaccine …
GeoVax Labs, Inc. receives positive Scientific Advice from the EMA for its GEO-MVA vaccine, streamlining the path to market authorization in the EU and potentially accelerating revenue generation.

9. GeoVax, Inc.
geovax.com
Home – GeoVax, Inc.
Aids Vaccine Research – GeoVax, Inc.
10. GeoVax, Inc.
geovax.com/investors/pres…
GeoVax Highlights Critical Role of Multi-Antigen COVID-19 …
With COVID-19 continuing to mutate and over 40 million immunocompromised Americans still at risk, GeoVax’s GEO-CM04S1 vaccine offers potential broader, more durable protection through a unique multi-antigen approach. ATLANTA, GA — July 3, 2025 —
11. Yahoo
finance.yahoo.com/news/geovax-hi…
GeoVax Highlights the Role of MVA-Based Vaccines in Advancing …
Recent Studies Support Diversified Vaccine Strategies, Aligning with GeoVax’s MVA-Based GEO-CM04S1 for Enhanced Protection, Particularly for Immunocompromised Populations ATLANTA, GA – February 24, 2025 (NEWMEDIAWIRE) – GeoVax Labs, Inc. (Nasdaq: GOV…

12. GeoVax, Inc.
geovax.com/component/rsbl…
GeoVax Highlights the Role of MVA-Based Vaccines in Advancing …
Recent Studies Support Diversified Vaccine Strategies, Aligning with GeoVax’s MVA-Based GEO-CM04S1 for Enhanced Protection, Particularly for Immunocompromised Populations ATLANTA, GA, February 24, 2025 – GeoVax Labs, Inc. (Nasdaq: GOVX), a clini
13. GeoVax, Inc.
geovax.com/investors/pres…
GeoVax Reports First Quarter 2025 Financial Results and Provides …
COVID-19 vaccine program progressing with additional data evaluating GEO-CM04S1 as booster to mRNA vaccines in healthy adults expected in second quarter of 2025 Clinical evaluation of GEO-MVA, vaccine candidate for protection against Mpox
14. Zacks Investment Research
zacks.com/stock/research…
What is the current Price Target and Forecast for GeoVax Labs (GOVX)
Find out the current price target and stock forecast for GeoVax Labs (GOVX)
15. MarketBeat
marketbeat.com/stocks/NASDAQ/…
GeoVax Labs (GOVX) Stock Forecast and Price Target 2025
GOVX’s current price target is $8.88. Learn why top analysts are making this stock forecast for GeoVax Labs at MarketBeat.

16. marketwatch
marketwatch.com/investing/stoc…
GeoVax Labs Inc. Analyst Estimates – GOVX – MarketWatch