Oil Prices, Tech Stocks, NVIDIA Surge On Otherwise Calm Monday – $AAPL $BCOM $EPRX $MCD $MSFT $NVDA $VIX Rise!

Global stock markets edged higher on Monday, August 12, 2024, while the yen slipped, setting the stage for a week filled with crucial economic data releases that could shape market sentiment and investor decisions. The S&P 500 barely budged, closing less than a point above its previous level, while the Dow Jones Industrial Average dipped .4% and the Nasdaq Composite inched up .2%. Only three S&P 500 sectors finished in positive territory, with information technology leading the pack at .9% growth. Energy stocks got a boost from a 4.1% jump in oil prices, reaching $79.91 per barrel, fueled by geopolitical tensions following the death of Hamas political leader Ismail Haniyeh. Meanwhile, the 10-year and 2-year Treasury note yields both decreased by three basis points, settling at 3.91% and 4.02% respectively. The small caps on the Russell 2000 closed at 2,062.08 (-.91%), while there iShares Micro-Cap ETF (IWC) dropped by .58% closing at $113.82. The Volatility Index (VIX), Wall Street’s “fear gauge,” closed at $20.71, +1.67% after hitting an intraday high of $21.13.
Tech Sector Gains
The information technology sector emerged as the day’s top performer, with a .9% rise driven by gains in tech giants. Apple (AAPL) saw a .7% increase, Microsoft (MSFT) edged up .2%, while NVIDIA (NVDA) surged an impressive 4.1% as stories of how generative AI is accelerating a learning curve & enabling human-like machines to pick up new tasks in real time surfaced on Monday Broadcom (AVGO) also contributed to the sector’s strength with a modest .24% gain. This tech rally helped offset losses in other areas, particularly the real estate and communication services sectors, which both declined by .6%. The strong performance of tech stocks highlights their continued importance in driving overall market movements, even in a relatively muted trading session.
Inflation Expectations Steady
According to the New York Fed’s Survey of Consumer Expectations, inflation expectations remained stable in July, with median one- and five-year-ahead projections holding steady at 3.0% and 2.8% respectively. Notably, the median three-year-ahead inflation expectation dropped by 0.6 percentage points to 2.3%, marking the lowest level since the survey’s inception in June 2013. This decline in longer-term inflation expectations could potentially influence future monetary policy decisions and market sentiment.
Treasury Budget Deficit
July’s Treasury Budget revealed a deficit of $243.7 billion, surpassing the $220.8 billion deficit from the same period last year. This financial gap resulted from government outlays of $574.1 billion outpacing receipts of $330.4 billion. The key takeaway from this report is the U.S. government’s persistent struggle with large budget deficits, driven significantly by net interest costs that now exceed defense spending. This trend underscores the ongoing fiscal challenges faced by the nation and may have implications for future economic policies and market dynamics.
McDonald’s $5 Meal Deal
McDonald’s (MCD, $269.46, +.58%) $5 meal deal is attracting budget-conscious diners, but the fast-food giant faces long-term challenges in maintaining its position as a value leader amid fierce competition and changing consumer preferences. Read more about this here.
VP Watchlist Updates

Eupraxia Pharmaceuticals (EPRX, $2.72, +1.12%) is a clinical-stage biotechnology company focused on the development of locally delivered, extended-release products that have the potential to address therapeutic areas with high unmet medical need. The Company strives to provide improved patient benefit and has developed technology designed to deliver targeted, long-lasting activity with fewer side effects. DiffuSphere™, a proprietary, polymer-based micro-sphere technology, is designed to facilitate targeted drug delivery, with extended duration of effect, and offers multiple, highly tuneable pharmacokinetic (PK) profiles. This investigational technology can be engineered for use with multiple active pharmaceutical ingredients and delivery methods.
On Aug. 2, Eupraxia announced entry into a new C$12 million convertible debt facility. Under the Convertible Debt Facility, Yabema Capital Limited and other current Eupraxia shareholders (together, the “Lenders”) will make available for drawdown an aggregate amount of C$12 million for a period of 120 days following entry into the agreement. The decision to draw on the facility within 120 days of closing is at the discretion of Eupraxia and is subject to the full and final release of the SVB Facility (as defined below), originally agreed to on June 21, 2021. The aggregate unpaid principal amount and any accrued and unpaid interest thereon will be convertible at each individual lender’s discretion into Eupraxia common shares (the “Common Shares”), at a conversion price equal to C$4.84375 per Common Share. The conversion is further subject to certain threshold limitations with respect to each lender’s aggregate ownership of the Common Shares. “The new convertible debt facility provides an important source of additional funding from long term, supportive investors, and creates greater stability to Eupraxia’s cap structure as we continue to advance our clinical programs in eosinophilic esophagitis and osteoarthritis,” said Dr. James Helliwell, Chief Executive Officer of Eupraxia. The Convertible Debt Facility is subject to final approval of the Toronto Stock Exchange.

Dr. James Helliwell, CEO of Eupraxia Pharmaceuticals (NASDAQ: EPRX)
On July 9, a Tribe Public CEO Presentation and Q&A Webinar Event titled “Exploring The Rapid Rise Of Osteoarthritis” was held with James A. Helliwell, MD, Director and Chief Executive Officer of Eupraxia Pharmaceuticals (NASDAQ: EPRX). The event video can now be viewed at the Tribe Public YouTube Channel!
 Shares of Lantern (LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug program, closed at $3.86, -1.15%.
Shares of Lantern (LTRN), an artificial intelligence (“AI”) company developing targeted and transformative cancer therapies using its proprietary RADR® AI and machine learning (“ML”) platform with multiple clinical stage drug program, closed at $3.86, -1.15%.
On August 9, announced operational highlights and financial results for the second quarter 2024, ending June 30, 2024 highlighting the following:
- Active clinical trials across three AI-guided drug candidates with additional ADC-based preclinical molecules in evaluation for development.
- Preliminary patient data and clinical readouts for Phase 2 LP-300 Harmonic™ Trial released showing an 86% clinical benefit rate in the initial 7 patient safety lead-in cohort.
- Issued a Certificate of Patent by the Japanese Patent Office directed to Lantern Pharma’s drug candidate LP-284, including claims covering the new molecular entity.
- Phase 1 clinical trials for both synthetic lethal drug candidates, LP-184 and LP-284, continue to advance with no dose-limiting toxicities observed in any of the patient cohorts enrolled and over 40 patients dosed to-date.
- Achieved significant advancement towards key milestone in the development of molecular diagnostic for use with drug candidate LP-184 in future oncology clinical trials to improve patient selection and stratification.
- Launched strategic drug development collaboration leveraging our AI platform, RADR®, with Oregon Therapeutics to optimize the development of first-in-class drug candidate XCE853 – a potent inhibitor of cancer metabolism.
- Starlight Therapeutics, a wholly owned subsidiary of Lantern Pharma focused on CNS and brain cancers advanced with initiating site selection and feasibility for a Phase 1B/Phase 2 trial in recurrent GBM with drug candidate, STAR-001.
- Launched Webinar Wednesdays, a webinar series that focuses on the areas of artificial intelligence and oncology drug development with leading physicians, scientists and Lantern collaborators.
Approximately $33.3 million in cash, cash equivalents, and marketable securities as of June 30, 2024.
On August 7, Lantern announced a significant advancement demonstrating the preclinical synergy of LP-184 with checkpoint inhibitors and the ability of LP-184 to resensitize tumors that have become non-responsive to Anti-PD1 therapies. The company will be presenting preliminary data from the recent work done in conjunction with Drs. Yong Du and Shiaw-Yih (Phoebus) Lin at MD Anderson at The Immuno-Oncology Summit 2024 in Philadelphia.
The data will be presented in the form of poster entitled, LP-184, a Novel Acylfulvene, Sensitizes Immuno-Refractory Triple Negative Breast Cancers (TNBCs) To Anti-PD1 Therapy by Affecting the Tumor Microenvironment, (assigned Poster # P17). The poster highlights the following key points:
-
LP-184 seems to potentiate anti-PD1 response in a mouse model of TNBC that is non-hypermutated and resistant to immunotherapy in the absence of LP-184.
-
LP-184 can potentially transform immunologically “cold” tumors (non-responsive to IO therapies) into “hot” tumors (responsive to IO therapies) by modulating T cell activity in the tumor microenvironment and inducing a replication stress response defect.1
-
LP-184 seems to reshape the tumor microenvironment (TME) by significantly reducing the amount of M2 macrophages – which are associated with tumor drug resistance, tumor cell proliferation and are involved in helping the tumor cells escape immune cell death2.
-
LP-184 combined with an anti-PD1 agent elicited a greater anti-tumor response than monotherapies in mouse TNBC tumors that are non-hypermutated and resistant to immune checkpoint inhibitors
LP-184 is being investigated in an ongoing first-in-human Phase 1 trial (NCT05933265) in advanced recurrent solid tumors to establish a maximum tolerated dose and assess its overall safety and suitability in more targeted cancer indications, including TNBC.
Immunotherapy with checkpoint inhibitors (CPI) account for nearly $48 billion in sales annually according to Grand View Research and has profoundly changed the landscape of treatment in oncology since their introduction by providing outstanding durable responses and potential long-term remission in a significant proportion of cancer patients.3 Treatments are now approved for more than thirty cancer indications including melanoma, lung, colon, renal, urothelial, gastric, liver, lymphoma, head and neck but only a minority of patients benefit (10% to 50% depending on the stage and site of the tumor) and often patients will be non-responsive to CPI.
“Our drug-candidate, LP-184 has shown very promising preclinical evidence supporting its role in immuno-oncology to help patients improve response and durability of response to IO therapies. This work in collaboration with MD-Anderson supports our initial AI-driven hypothesis regarding the role of LP-184 to synergize with PD1 and PDL1 drugs and potentially improve the lives of a greater number of cancer patients globally. We look forward to developing combination drug studies and clinical trials with LP-184 and checkpoint inhibitors,” said Lantern Chief Scientific Officer, Kishor Bhatia, PhD, FRCP.
The entirety of the data and poster to be presented at The Immuno-Oncology Summit 2024 in Philadelphia will be available on the Lantern website after 6pm Eastern today, August 7th 2024.
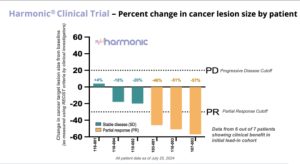
On August 5, Lantern Pharma announced promising preliminary results from its Phase 2 HARMONIC™ clinical trial, evaluating LP-300 in combination with standard chemotherapy for never-smokers with advanced non-small cell lung cancer (NSCLC). As reported earlier today, August 5, the study has shown an impressive 86% clinical benefit rate in the initial patient group, offering hope for a patient population with limited treatment options. The proportion of never-smoking patients with non-small cell lung cancer (NSCLC) has been significantly increasing globally over the past 30 years, from 15% in the 1970s to 33% in the 2000s. The high proportion of never smokers with NSCLC in East Asian countries is of particular note with Japan estimated to be 33 to 40% of new cases and Taiwan at over 50% of new cases. Lantern has received regulatory approval to initiate the LP-300 clinical trial in multiple Asian countries, and has started activation of sites in Japan and Taiwan, including the National Cancer Center in Tokyo, a globally recognized center of cancer research excellence. Read the balance of the story.
Panna Sharma, CEO of Lantern Pharma was interviewed recently on the ‘Today In Nashville’, a program hosted by Carole Sullivan and associated with Nashville’s WSMV 4, an NBC affiliate. Watch it here to learn more.
 Shares of Indaptus Therapeutics, Inc. (Nasdaq: INDP) closed at $1.71, -1.18% and is up +15.79% at $1.98 in the aftermarket. Indaptus is a company with the ability to harness both the body’s innate and adaptive immune responses, believes that they are uniquely positioned to revolutionize the treatment of cancer and certain infectious diseases. Indaptus Therapeutics has evolved from more than a century of immunotherapy advances. The Company’s novel approach is based on the hypothesis that efficient activation of both innate and adaptive immune cells and pathways and associated anti-tumor and anti-viral immune responses will require a multi-targeted package of immune system-activating signals that can be administered safely intravenously (i.v.). Indaptus’ patented technology is composed of single strains of attenuated and killed, non-pathogenic, Gram-negative bacteria producing a multiple Toll-like receptor (TLR), Nucleotide oligomerization domain (Nod)-like receptor (NLR) and Stimulator of interferon genes (STING) agonist Decoy platform. The products are designed to have reduced i.v. toxicity, but largely uncompromised ability to prime or activate many of the cells and pathways of innate and adaptive immunity. Decoy products represent an antigen-agnostic technology that have produced single-agent activity against metastatic pancreatic and orthotopic colorectal carcinomas, single agent eradication of established antigen-expressing breast carcinoma, as well as combination-mediated eradication of established hepatocellular carcinomas and non-Hodgkin’s lymphomas in standard pre-clinical models, including syngeneic mouse tumors and human tumor xenografts.
Shares of Indaptus Therapeutics, Inc. (Nasdaq: INDP) closed at $1.71, -1.18% and is up +15.79% at $1.98 in the aftermarket. Indaptus is a company with the ability to harness both the body’s innate and adaptive immune responses, believes that they are uniquely positioned to revolutionize the treatment of cancer and certain infectious diseases. Indaptus Therapeutics has evolved from more than a century of immunotherapy advances. The Company’s novel approach is based on the hypothesis that efficient activation of both innate and adaptive immune cells and pathways and associated anti-tumor and anti-viral immune responses will require a multi-targeted package of immune system-activating signals that can be administered safely intravenously (i.v.). Indaptus’ patented technology is composed of single strains of attenuated and killed, non-pathogenic, Gram-negative bacteria producing a multiple Toll-like receptor (TLR), Nucleotide oligomerization domain (Nod)-like receptor (NLR) and Stimulator of interferon genes (STING) agonist Decoy platform. The products are designed to have reduced i.v. toxicity, but largely uncompromised ability to prime or activate many of the cells and pathways of innate and adaptive immunity. Decoy products represent an antigen-agnostic technology that have produced single-agent activity against metastatic pancreatic and orthotopic colorectal carcinomas, single agent eradication of established antigen-expressing breast carcinoma, as well as combination-mediated eradication of established hepatocellular carcinomas and non-Hodgkin’s lymphomas in standard pre-clinical models, including syngeneic mouse tumors and human tumor xenografts.
On August 8, Indaptus announced that it has entered into securities purchase agreements with investors, including an officer of Indaptus, for the issuance and sale of an aggregate of 1,643,837 of its shares of common stock. In a concurrent private placement, Indaptus has also agreed to issue and sell unregistered warrants to purchase up to an aggregate of 1,643,837 of its shares of common stock. The combined effective purchase price for each share of common stock and associated warrants is $1.825. The warrants will have an exercise price of $1.70 per share, will be immediately exercisable upon issuance and have a term of five years from the date of issuance. The closing of the offering is expected to take place on or about August 8, 2024, subject to the satisfaction of customary closing conditions. Paulson Investment Company, LLC is acting as the exclusive placement agent in connection with the offering. The gross proceeds to Indaptus from the offering are expected to be approximately $3.0 million, before deducting the placement agent’s fees and other offering expenses payable by Indaptus. Indaptus intends to use the net proceeds from the offering to fund its research and development activities and for working capital and general corporate purposes.

Shares of ADT Inc. (ADT), a leading provider of monitored security and automation solutions for residential and small business customers in the United States and Canada, closed at $7.17, -.69% after establishing a new 52-wk high of $7.92 during intraday trading recently.
On Aug. 1, ADT reported its second quarter results for 2024 that read like a thrilling spy novel, complete with mysterious numbers and covert operations. The company reported a 3% increase in total revenue, reaching $1.2 billion – apparently, securing homes is more lucrative than ever in our paranoid future. Their recurring monthly revenue (RMR) grew by 2% to $355 million, proving that once ADT gets its foot in your door, it’s there to stay
The company boasted “strong customer retention” with a gross revenue attrition of 12.9%, which in ADT speak means they’re only hemorrhaging about 1 in 8 customers. Their “revenue payback” sits at 2.2 years, suggesting it takes that long for customers to stop regretting their decision to sign up.
In a plot twist worthy of a summer blockbuster, ADT’s GAAP income from continuing operations dropped by $54 million. But fear not, shareholders! Their “adjusted” income increased by $3 million. It seems ADT has mastered the art of financial alchemy, turning red numbers green faster than you can say “creative accounting”.
CEO Jim DeVries, channeling his inner motivational speaker, declared that ADT’s success is “powered by our employees’ dedication to the proposition that every second counts.” One can only imagine the intense pressure of working in an environment where bathroom breaks are timed to the millisecond.[

Modular Medical, Inc. (NASDAQ: MODD, $1.55, -1.27%), is a development-stage, insulin delivery technology company seeking to launch the next generation of user-friendly and affordable insulin pump technology. Using its patented technologies, the company seeks to eliminate the tradeoff between complexity and efficacy, thereby making top quality insulin delivery both affordable and simple to learn. Their mission is to improve access to the highest standard of glycemic control for people with diabetes taking it beyond “superusers” and providing “diabetes care for the rest of us.” Modular Medical was founded by Paul DiPerna, a seasoned medical device professional and microfluidics engineer. Prior to founding Modular Medical, Mr. DiPerna was the founder (in 2005) of Tandem Diabetes and invented and designed its t:slim insulin pump. More information is available at https://modular-medical.com.
In a move that’s sure to spice up the insulin pump manufacturing scene, Modular Medical announced today, August 7, that it is packing its bags and heading south of the border. The company has begun transferring its pilot line manufacturing operations to a Phillips Medisize site in Queretaro, Mexico. Trusted for nearly 60 years, Phillips Medisize, a Molex company, is a global leader in front-end design, development and manufacturing solutions for highly regulated industries — pharma, in vitro diagnostics, med tech, consumer, automotive and defense. Operating as a single integrated collaborator, they help their customers reduce risk and achieve product realization quickly and efficiently. Their innovation, quality and reliability enhance the lives of millions of people around the world. Modular Medical’s relocation is happening alongside the FDA’s ongoing 510(k) review of the MODD1 Insulin Delivery System. Chief Operating Officer Kevin Schmid confidently predicts that the manufacturing operation will be validated and ready for human-use production by early next year. The MODD1 system will be manufactured in a clean room in Queretaro, while the printed circuit board assembly will be crafted in Guadalajara, showcasing a truly international effort in diabetes care technology. Phillips Medisize, a Molex company, has been a crucial partner in developing Modular Medical’s platform product, supply chain, and manufacturing operations. Their collaboration extends beyond basic manufacturing, providing expertise in injection molding, packaging, electronics design, and assembly operations. This partnership has been instrumental as Modular Medical transitions from pre-commercial production to high-volume device manufacturing. The collaboration leverages Phillips Medisize’s global and diversified supplier base, enabling the design and development of manufacturing capabilities specifically tailored for the MODD1 Insulin Delivery System. Phillips Medicine was founded in 1964 in Phillips, WI, employs 6 thousand employees worldwide, has 24 manufacturing locations in 11 countries, including 5 R&D centers globally 2.5M+ square feet of manufacturing space, & maintains Class 7 & 8 cleanrooms and tool building sites. The insulin pump market is experiencing robust growth, with a valuation of USD 6.05 billion in 2023 and a projected compound annual growth rate (CAGR) of 8.22% from 2024 to 2030. This expansion is driven by technological advancements, increasing diabetes prevalence, and a growing elderly population. Key players in the market include Medtronic (MDT), Abbott Laboratories (ABT), Roche Diagnostics & Tandem Diabetes Care, Inc (TNDM). The global diabetes care devices market is expected to reach USD 52.34 billion by 2029, growing at a CAGR of 12.22% from 2024 to 2029. This growth is fueled by rising healthcare spending, increased awareness about diabetes management, and the development of innovative devices like continuous glucose monitors and automated insulin delivery systems.
Gain Further Insights From The CEO of Modular Medical…
On Friday, August 9, our sister organization, Tribe Public (www.TribePublic.com), hosted a brief CEO Presentation and Q&A Webinar-based event with Jeb Besser, CEO of Modular Medical (NASDAQ: MODD). The event is titled “Diabetes Care Innovator Modular Medical Takes Next Steps.”
Quote of the Day
Economic Reports
On Monday, July’s Treasury Budget revealed a deficit of $243.7 billion, surpassing the $220.8 billion deficit from the same period last year. This financial gap resulted from government outlays of $574.1 billion outpacing receipts of $330.4 billion. The key takeaway from this report is the U.S. government’s persistent struggle with large budget deficits, driven significantly by net interest costs that now exceed defense spending. This trend underscores the ongoing fiscal challenges faced by the nation and may have implications for future economic policies and market dynamics.
Videos
Post View Count : 501















